On September 12, 2012, Governor Edmund G. Brown Jr. signed Assembly Bill 685, making California the first state in the United States of America to legislatively recognize the fundamental human right to water. Now in the California Water Code as Section 106.3, the State further recognizes that “every human being has the right to safe, clean, affordable, and accessible water adequate for human consumption, cooking, and sanitary purposes”. The “human right to clean water” is a sentiment echoed worldwide, but is an increasingly more difficult and expensive goal to attain; especially with explosive population growth and the ongoing discovery of emerging contaminants.
America’s water treatment and delivery infrastructure is aging and outdated, and central plant operators are doing the very best they can within the confines of limited budgets, outdated equipment, and arcane regulations.
Considering the catastrophic water-quality failure events that are so frequently highlighted during our 24-hour news cycle, more and more Americans are realizing that they need to take responsibility upon themselves to improve the taste, odor, appearance, and sometimes even the safety of centrally-supplied water. This makes sense, since such a small amount of municipal water is consumed by humans, and it is so prohibitively expensive to address every single potential contaminant or waterborne threat comprehensively at the city level.
Waterborne microorganisms range in size from extremely small viruses in the submicron range to relatively large cysts than can approach 50 micron in diameter. Pathogenic microorganisms can occur naturally in lakes, streams, reservoirs, and most surface water sources. Even groundwater supplies are not immune, since the existence of subterranean bacteria has been definitively proven along with the ability of enteric viruses and other organisms to leach into groundwater from land application or burial of sewage sludge and other residential and industrial wastes.
One hundred and fifty years ago, much of the USA’s water supply was teeming with various forms of aquatic organisms including coliforms, bacteria, viruses, and protozoa. Waterborne diseases, such as cholera, typhoid, and dysentery, were a serious public health problem, and they are still major concerns in third-world nations where over a billion people lack clean drinking water and almost two billion lack adequate sewage distribution and processing systems.
The United States Environmental Protection Agency ranks drinking water pollution as one of the top environmental threats to health. Credible estimates suggest that only half of waterborne disease outbreaks in community water systems and about one third of those in non-community systems are ever formally detected, investigated, or even reported. Microbes in tap water may actually be responsible for as many as 30% of gastrointestinal illness in the United States. Recent studies indicate that there is a far greater waterborne transmission pathway for viral activity than previously believed.
Chlorine-based disinfection saves lives
There are nearly 250,000 public water supply systems in the United States, serving everything from the smallest towns to major metropolitan areas. Approximately 90% of the US population currently receives their water through community water systems, with everyone else using private wells or other individually controlled supplies.
Chlorine and Chloramine are currently used by over 98 percent of all U.S. water utilities as part of their treatment and disinfection process. Almost 80% of utilities currently use Chlorine instead of Chloramine.
The typical municipal water treatment process involves a series of different steps. Some of the major steps include flocculation and coagulation, sedimentation, filtration and disinfection. Chlorination is typically performed at several stages of the treatment process. On surface water supplies, chlorine will usually be introduced in the initial stages to combat algae and other aquatic life that could interfere with treatment equipment and subsequent stages in the process.
The chlorination stage that we’re most interested in occurs as the final treatment step after the other major cleaning processes, where the concentration and residual content of the chlorine can be closely monitored and controlled. Chlorine remains in the water when it is distributed to homes and businesses, retaining some of its ability to continue killing and reacting with undesirable contaminants in the water and distribution system.
Chlorination can deactivate microorganisms through a variety of mechanisms, such as oxidative damage to cellular membranes, inhibition of enzymes, destruction of nucleic acids, and even other mechanisms that are not fully understood. The effectiveness of any chlorination process depends upon a variety of factors, including chlorine concentration, contact time, water temperature, pH value, level of turbidity, and other interfering factors.
Chlorination is not 100% effective against all waterborne contaminants, and undesirable byproducts will be formed in the treated water, but it is undoubtedly the cheapest, most effective way to disinfect water that is stored, processed and distributed to homes and businesses; protecting us all from many deadly and undesirable waterborne contaminants.
Chloramine
Chloramines are derived from the combination of Chlorine and Ammonia, where chlorine is substituted for one or more hydrogen molecules in the compound. There are three known Chloramine species:
- Monochloramine (“Chloroamine”, NH2Cl) – The most effective biocide
- Dichloramine (NHCl2), and
- Nitrogen trichloride (NCl3)
Chloramines can form spontaneously in water, or be deliberately formed at the municipal level, since a growing minority of central providers actively chose chloramine as their disinfectant technology.
The various species of chloramine can rapidly shift from one form to another throughout the distribution system. The predominant species depends on pH, temperature, dissolved oxygen, carbon dioxide, organics in the water, and the instantaneous chlorine to ammonia ratio.
Monochloramine is less reactive with other organics in water than free chloramine, so it will stay active for longer in the water and form significantly fever trihalomethanes and other undesirable “chlorine related” byproducts – this makes it an enticing choice for many providers.
Other undesirable disinfection byproducts can form though, such as toxic halonitriles (cyanogen chloride), halonitromethanes (chloropicrin) and other nitrogen-rich compounds. Some of these compounds can endanger human health.
Chloramines are all respiratory irritants with trichloramine being the most problematic. Chloramine is a major contributor to the corrosion of copper and brass, as well as degradation of certain rubber compounds. Nitrification is also a concern with Chloramine; this is a microbial process where one type of nitrifying bacteria oxidizes ammonia to produce nitrite (NO2–), and another will oxidize nitrite to produce nitrate (NO3–). The problem is greatest when temperatures are warm and water is allowed to stagnate in the distribution system (low water usage).
The limited adoption of Chloramination at a municipal level in the USA speaks to the complexities associated with it, and the concerns that many have about byproducts, increased corrosivity towards metals and Nitrification.
Chloramines are challenging to remove with “regular” activated carbon, since it takes more than just the simple reducing action of activated carbon. I’ve had the very best results with various catalytic carbon blends depending on what other contaminants are in the water. Bear in mind that nitrification can occur after your de-chloramination system; this can be problematic, especially if infants or those with compromised immune systems will be using the water.
Disinfection byproducts
Epidemiological studies have related exposure to chlorination byproducts with birth defects, pregnancy complications, certain cancers like bladder, rectal and kidney (recent studies suggest there might also be a causal relationship between chlorine byproducts and breast cancer in men and women), respiratory stress, eye irritation, skin damage, headaches and fatigue.
Traditionally, the risk of chlorine disinfection byproducts has been downplayed by legislators and industry, since the risk of non-chlorination is far greater. In fact, the World Health Organization (WHO) recently stated – “the risk of death from pathogens is at least 100 to 1000 times greater than the risk of cancer from disinfection by-products (DBPs) {and} the risk of illness from pathogens is at least 10,000 to 1 million times greater than the risk of cancer from DBPs“. The consumer is being told that they must effectively choose between illness and/or death from waterborne disease and microorganisms, or a steady decline in quality of life from the permanent damage caused by chlorine compounds and the inevitable byproducts of disinfection.
Today’s affordable water quality improvement technologies give consumers a much better option: – Disinfect and protect the water with industrial chemicals like chlorine at the municipal level to keep it as safe as possible until it reaches the home, and then reduce or completely remove the chlorine, disinfection byproducts, and other contaminants; effectively enjoying the best of both worlds.
Anatomy of a whole house carbon filter
There are numerous carbon-based options available to protect your client and their family from chlorine tastes and odors, pesticides, herbicides, emerging contaminants, and various disinfection byproducts. As a water treatment professional, your primary responsibility is to provide your clients with the very best water at an affordable price in an environmentally responsible manner.
The scope of this article is specific to chlorine/chloramine tastes and odors, so if you’re planning on addressing lead, pesticides, herbicides, or pharmaceutical byproducts consult with your equipment manufacturer before making claims on what your carbon filter can actually do. Not all carbons work the same, especially with complex organics and varying influent water chemistries.
The simplest and cheapest option is a replaceable POE carbon cartridge, but it has a major downside – reduced flow and pressure. Seasoned professionals will recommend a tank-based Point-of-Entry (POE) system that meets the consumers’ budget and performance requirements and can provide the necessary flow and longevity.
Whole house carbon filters can be self-backwashing, non-backwashing upflow, or non-backwashing downflow. Each has distinct advantages and disadvantages.
Non-backwashing downflow – A simple tank with in/out head and distribution system. Usually loaded with gravel and carbon media. Water enters at the top of the tank, moves downwards through the media column and then up the riser. This compacts the bed, traps certain sediment and maximizes contact time with the media. The disadvantage to this type of system is that the media column will be that unless it is properly prefilters, it will eventually become fouled with sediment and fines resulting in unacceptable pressure-drop and channeling. Scheduled periodic maintenance is critical on these systems.

Non-backwashing upflow (Figure 1.) – A simple tank with in/out head and distribution system. Usually loaded with gravel, bacteriostatic media and carbon media. Water enters downward through the riser, moves upwards through the media column and then exits at the top of the tank. While the upflow service protects the media from sediment fouling, it minimizes the effective contact time with the media and often allows for bleedthrough of contaminants.
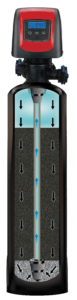
Backwashing (Figure 2.) – A simple tank with self-backwashing control head (Preferably computerized with a flowmeter) and distribution system. These types of systems are typically loaded with gravel, bacteriostat, sediment filtration media, and activated carbon media. Water enters at the top of the tank, moves downwards through the media column and then up the riser. This compacts the bed, traps certain sediment and maximizes contact time with the media. After a certain number of gallons have been processed, or after a designated calendar interval, the system backwashes to reclassify the media, purge trapped sediment, gases and media fines while minimizing the potential for biofilm growth.
Completing the treatment train
Whole house carbon systems are frequently installed along with other treatment technologies, such as Water Softeners, Ultrafilters, and Ultraviolet disinfection systems. Since I specify water softeners with highly chlorine-resistant resin, I usually recommend whole house carbon filter be installed after the water softener and before ultrafiltration or UV to ensure the highest quality of water downstream. Naturally, there are exceptions to every rule, so consult with your local Master Water Specialist and your equipment manufacturer when selecting any water quality improvement solution.
Installation, Startup, and Maintenance
Regardless of the system/s that you install for your client, it is important to apply the appropriate level of care and safe handling when working with systems that incorporate activated carbon. Here are some helpful guidelines:
- Carbon-based systems should remain sealed until the absolute last moment to avoid absorption of airborne odors or other contaminants.
- Air must be carefully purged from the carbon bed. The ideal method is to soak the carbon in warm sanitary water and give the carbon sufficient time to purge entrained air – this can sometimes take 72-hours or more; consult with your equipment vendor to ensure that you are using the very best practices.
- Carbon-based systems as well as the downstream plumbing should be disinfected upon startup to minimize biofilm formation and other bacterial activity downstream.
- Media should not be allowed to sit still for too long, I recommend at least a weekly backwash to minimize the development of biofilm.
- Systems should be periodically inspected and disinfected.
- Replace or augment the carbon and other treatment media on a regular maintenance schedule as recommended by the equipment manufacturer and per industry best practices.
Sustainability and Certification
As professionals, we need to ensure that every aspect of our water quality management and improvement process is as environmentally responsible, sustainable, and as renewable as possible. The WQA along with ASPE and ANSI have developed Sustainability standards to:
- Encourage more strategic participation among product manufacturers for the advancement of sustainable products and business practices through improvements in the areas of product design, manufacture and production site management, distribution, disposal, etc.;
- Allow for evaluation of certification based on product categories, as well as the environmental performance of entire production facilities, as opposed to just evaluating all the details on a product-by-product basis.
- Reduce organizational burden and cost in pursuing certification of products, and reduce business risk from internal competition among similar products by the same manufacturer.
- Reduce regulatory expense and risk, reduce production costs, and potentially preempt mandatory regulatory initiatives through the adoption of a voluntary management based approach to sustainability issues throughout the industry.
The WQA/ASPE/ANSI S-802: Sustainable Activated Carbon Media for Drinking Water Treatment covers raw activated carbon media products. Certification to this standard covers the sustainability of material sourcing, transportation, processing, distribution, and end-of life planning to ensure a minimal environmental impact and preserving the Health and Safety of workers throughout the supply chain.
Talk to your vendor/s and demand that they use Sustainable Carbon in your systems, it’s the responsible and ethical thing to do.
In addition to the sustainability of products, the actual performance of systems can be measured against NSF standard such as NSF 42 for aesthetic effects. Bear in mind that dechlorination systems are frequently customized for a specific project, and will not necessarily be certified as an actual system.
Activated Carbon is a powerful tool in your water treatment arsenal, and you’d be wise to learn as much as possible about this valuable resource to ensure that you continue to provide you clients with the very best water at the most affordable price.
Image Credit: The Innovative Water Project
Special thanks to Stuart Mann, Sustainability Certification Manager at the Water Quality Association.
Glossary
Activated Carbon – Carbonaceous material processed to allow the development of high density pores that increase the net surface area available for absorption, adsorption, or chemical reactions. Activated carbons commonly used in the water treatment industry are typically derived from coconut shell, bone, and other organic feedstocks.
Catalyst – A substance which increases the rate of a chemical reaction without necessarily expending itself or becoming an actual part of the reaction.
Catalytic (Surface Modified) Carbon – Activated carbon that has been modified through a (usually proprietary) process to allow the Activated Carbon to possess catalytic activity. The catalytic activity of carbon can be measured by the rate at which it decomposes hydrogen peroxide. The peroxide number is typically represented as the time (in minutes) required to decompose a specific amount of Hydrogen peroxide (H2O2). The peroxide number also can be represented in terms of capacity to decompose a variable volume of hydrogen peroxide in a fixed time.
Trihalomethanes – Chemical Compounds where three of the four hydrogen atoms of methane are replaced by halogen atoms. Many trihalomethanes are used in industry and the home as solvents or refrigerants. THM’s are generally considered environmental pollutants and many are carcinogenic. The USEPA currently limits THM’s (chloroform, bromoform, bromodichloromethane, and dibromochloromethane) to 80 ppb in treated water. Levels at the plant are often lower than those at the tap, since chlorine continues to react with organics throughout the distribution system.
Haloacetic Acids – Carboxylic acids where a halogen atom replaces a hydrogen atom in acetic acid. Haloacetic acids in varying forms are common disinfection byproducts of chlorination.
Chloroform – A trihalomethane reagent/solvent, considered an environmental hazard. Chloroform is often inadvertently synthesized during the water treatment process when chlorine and related compounds are added to water. The US Department of Health and Human Resources National Toxicology Program’s eleventh report on carcinogens implicates chloroform as a human carcinogen; a designation equivalent to International Agency for Research on Cancer class 2A. It has been most readily associated with hepatocellular cancer. Chloroform once appeared as an ingredient in toothpastes, cough syrups, ointments, and other pharmaceuticals, and was banned in the USA as a consumer product ingredient in 1976.
Chloropicrin (Cl3CNO2) – A chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. Chloropicrin is also referred to as “PS” or nitrochloroform. Chloropicrin is toxic to humans, and can be absorbed through inhalation, ingestion, and dermal (skin) contact. It can be severely irritating to the lungs, eyes, and skin.
