 When selecting a softening resin for your particular application, consult carefully with the vendor to ensure the resin will actually meet your needs, not just the one that makes the most commission. Some dealers purchase pre-built systems while others assemble their own. This article shares a few important concepts about resin selection that will hopefully assist both camps. It is important to stress that my goal here is not to turn you into a systems engineer, just to look into our world to help make things a little clearer.
When selecting a softening resin for your particular application, consult carefully with the vendor to ensure the resin will actually meet your needs, not just the one that makes the most commission. Some dealers purchase pre-built systems while others assemble their own. This article shares a few important concepts about resin selection that will hopefully assist both camps. It is important to stress that my goal here is not to turn you into a systems engineer, just to look into our world to help make things a little clearer.
When I design systems, I select resins with rapid kinetics and a structured exchange matrix that allows for effective cleaning in a variety of adverse conditions with uniform performance across a wide variety of operating temperatures. Before even selecting a resin, I always consider the following:
- Service flowrate:
- Higher flowrates require deeper columns of resin to ensure adequate exchange.
- Overall columnar area should be designed to minimize pressure loss.
- Don’t oversize the bed, keep the service flow at least >2gpm/ft2 to prevent channeling
- Water temperature:
- Cold water increases pressure drop through an ion exchange column.
- Cold water significantly reduces ion exchange kinetics, requiring a deeper column to ensure adequate ion exchange.
- Warmer water requires a faster backwash to provide adequate bed expansion.
- Water pressure:
- Certain minimum water pressures are required to ensure adequate eduction of regenerant.
- Available water flow:
- The available water flow is not merely to satisfy the desired service flowrate, but also cleaning/regeneration cycles – especially on multiplex systems.
It is quite common to overlook the foregoing, since many designers are either uneducated, lazy, in a hurry, or simply underestimate the importance of the fundamentals. Once the fundamentals have been determined, then the astute designer will further ascertain the following:
- Amount of hardness being addressed:
- Higher hardness levels require deeper columns of resin to ensure adequate exchange.
- Higher hardness levels usually include higher levels of complicating and interfering ions.
- Higher hardness levels can cause scaling in regeneration assemblies and require cleaning agents that might damage resin
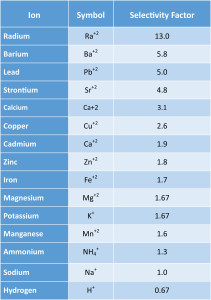
- Amount of interfering and complicating factors in the water:
-
-
- Softening resins have varying affinity for cations in water; generally, the higher the molecular weight of the ion, the higher the selectivity. Interfering ions will not only replace the exchange ion on the resin (sodium/potassium), but also any other ions that are less attractive to the resin.
- Organics and bacteria in water can foul ion exchange sites, significantly reducing ion exchange capacity, as well as potentially creating a home for other bacteria that might have negative health effects.
- Interfering factors such as iron and other metals will not only interfere with softening ability, but also potentially foul the resin.
-
-
- Water conductivity/TDS:
-
-
-
- Water conductivity has an effect on ion exchange success: the more conductive the water, the more interfering ions there are to attach onto exchange sites and potentially induce hardness leakage.
-
-
-
-
- Amount of acceptable hardness leakage:
- How much hardness is acceptable in the finished water? – Is this system serving a home, a laundromat or an expensive boiler system? Hardness leakage levels acceptable for a household system are completely unacceptable for a commercial/industrial system. The amount of acceptable hardness leakage will determine which resin is used, how much of it is used, how it will be regenerated and even how much regenerant salt is to be used for the regeneration.
- Amount of acceptable hardness leakage:
-
-
-
-
Armed with more knowledge, we can now begin the process of selecting an appropriate ion-exchange resin. As previously discussed, there a many varieties of softening resin available on the open market as well as a number of highly advanced proprietary softening media. Key to choosing a softening resin is understanding some of the terms used to describe it:
Capacity – The amount of contaminants the softening media can exchange under a set of standardized parameters (these can vary by manufacturer) in equivalent units (usually calcium carbonate). Capacities are also expressed at different salting levels, allowing design flexibility when balancing salt-efficiency with permissible hardness leakage.
Cross-linkage – Linear polymer chains bound within the matrix of the resin bead to give it structure and insolubility. Crosslinking materials can vary and have good or bad effects on capacity. Generally speaking, higher capacity resins tend to have lower capacities and kinetics, but there are definitely exceptions to this rule. Higher cross-linkage resins last longer in chlorinated water and increase in strength as the percentage of cross-linkage increases.
Size – Resin bead sizes vary. The size and uniformity of the bed will have a dramatic effect on net throughput, capacity and service hardness leakage. Regular resins are shipped within a Gaussian size distribution of 16-50 mesh. Smaller diameter, fine-mesh resin beads yield more capacity per volume, but at the expense of increased pressure drop through the bed and usually more sensitivity to oxidative damage.
Structure – The structure of an ion exchange resin describes how the resin is built. Gel resins are standard hard polymer beads, Macroporous resins are highly cross-linked with carefully engineered voids to allow fast interaction between water and exchange sites. Certain specialty structured matrix media leverage the best of both worlds to allow maximum ion exchange capacity, strength and resistance to osmotic shock during operation.
Uniformity – of Size is a benefit, since it provides for improved flow through the bed as well as uniform kinetics. Uniform beds regenerate faster with less salt and water.
Whole Bead Count – This refers to the percentage of resin beads that are whole or completely spherical in a sample. Expressed as a percentage, it is a good general indication of quality control during manufacture. Broken beads contribute to pressure drop through a bed and inevitable lead to channeling. Whole bead counts less than 90 percent are entirely unacceptable in new resins.
Now you have enough information to begin making informed decisions when consulting with your equipment manufacturer to ensure that you are always providing your customers with the best water at the very best price.
Glossary
Mesh – This sometimes ambiguous term refers to the size of a particle. In the ion exchange industry, we use the US Standard mesh, where 16 mesh is approximately 1,200 microns in diameter, 50 mesh is approximately 300 microns in diameter, and 100 mesh is 150 microns in diameter.
Regenerant – Chemical compound used to replenish ion exchange sites throughout the resin media. Sodium chloride or potassium chloride salts are commonly used to regenerate water softeners.
Regeneration – process of inducing a liquid solution of regenerant to the resin and forcing exchanged contaminants off of exchange sites through mass-action. Exchange sites are then occupied by ions from the regenerant.